The ROLO Kids and ROLO PreTeen studies are longitudinal follow-up studies of the original ROLO randomised control trial which assessed the impact of a low glycaemic index diet on birth weight, maternal glucose intolerance and gestational weight gain.
This study started with 800 pregnant women taking part from early pregnancy. Mothers and children from the ROLO study are followed up at 6 months, 2 years, 5 years, and 10 years of age in order to determine whether maternal nutrition and low GI diet in pregnancy impacts on maternal and child health in the long term.
As a natural progression from the ROLO study, Pregnancy Exercise and Nutrition Study with Smartphone Application Support : A Ransomised Controlled Trial (PEARS) was designed to assess the impact of a lifestyle intervention package, which consisted of a low glycaemic index (GI) diet and exercise prescription with smart phone app support, , on the incidence of gestational diabetes in an overweight and obese pregnant population. Women with a BMI of greater than 25 kg/m2 have a higher risk of developing gestational diabetes and a low glycaemic index diet in pregnancy has shown to lower glucose intolerance. This randomised controlled trial recruited 500 women. The addition of a specifically designed research smart phone app is novel and holds considerable potential to alter maternal behaviour in a positive way. An economic assessment of this intervention will allow assessment of its role within routine antenatal care.
The Probiotics in Pregnancy (ProP) study was a double-blind, placebo-controlled randomised trial that investigating the effects of a probiotic capsule intervention on maternal fasting glucose and other indices of maternal metabolism. The probiotics are well tolerated and amongst the obese pregnancies no impact was noted on maternal metabolic profiles. The impact of probiotics on women with a new diagnosis of gestational diabetes shows effect on maternal lipids.
The Trial of low dose aspirin with an Early Screening Test for preeclampsia and growth restriction (TEST) study is a large (n=500) multicentre, randomised controilled feasibility trial of aspirin in low risk pregnancy. This was the first drug trial in pregnancy in Ireland and it was very well received among low risk unselected women.
Microbe Mom is a new therapeutic research collaboration investigating:
Microbe Mom is a 4-way collaboration between:
Additional information can also be found on http://apc.ucc.ie/MicrobeMom/
The GetGutsy study is testing the effect of taking a probiotic on markers of metabolic health. Recruitment is currently ongoing.
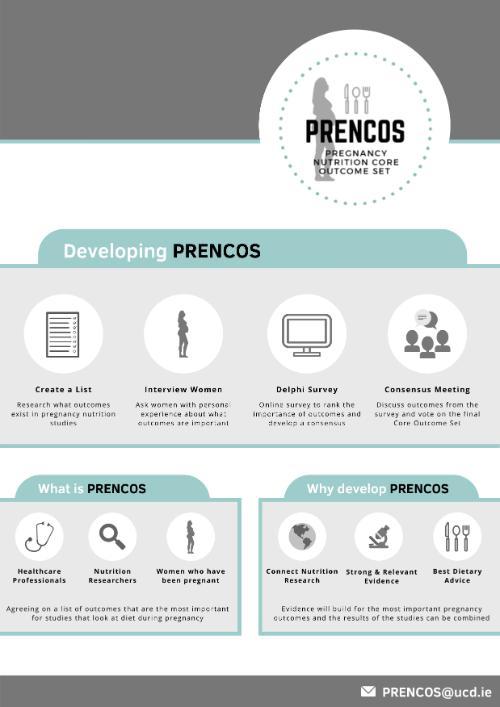
Pregnancy Nutrition Core Outcome Set (PRENCOS)
Preterm Birth continues to be an issue of global importance and has a major impact on both maternal and child health. Being born preterm can have a significant adverse affect on the health of an infant and this adversity ripples into childhood and adult life.
The Perinatal Research Centre at UCD has a dedicated research stream targeting issues relating to Preterm Birth. Our aim to investigate the causes of and treatment for Preterm Birth in order to improve outcomes for babies, their mothers and their families.
Our Preterm Birth Clinic is uniquely situated to deliver high calibre research in this area. We are fortunate to be staffed with a Sub Specialist Obstetrician, a dedicated Midwife, clinical and research fellows and have built links with our surgical and neonatal colleagues.
Our current focus is on the role of the vaginal microbiome in spontaneous Preterm Birth. This group have identified bacterial species whose deficiency is associated with Preterm Birth. We are currently enrolling for our randomised controlled trial of a bespoke Probiotic in women at risk of Preterm Birth.
A multifaceted m health and health coach supported intervention to reduce GDM in at risk women at NMH, Bristol, Granada and Melbourne commenced recruitment
A number of studies have been performed examining the interaction of vitamin D on maternal and fetal health.
This is an on-going multicentre study following up, at 2 and 5 years, children whose mothers were recruited, in pregnancy, with fetal growth on the 10th centile, in collaboration with Perinatal Ireland.
We are delighted that a smart phone app developed by the centre in collaboration with Dr Eileen O’Brien and Ms Sinead Curran at Dept of Dietetics at NMH was launched in 2021 for all pregnant women at NMH, nationally and internationally under name Hollestic, available on app store and free to download. It is based on the PEARS randomised controlled trial that found that an m-health lifestyle supported intervention resulted in less gestational weight gain, better sugar levels in mothers and less large for gestational infants. To date it has > 100,000 downloads, with the majority from outside Ireland. This is an excellent example of translation of research into a clinically useful tool for women attending National Maternity Hospital and globally
This is an ambitious multicentre randomised controlled trial to support breastfeeding amongst women with BMI > 25 which includes intensive antenatal and postnatal support.
Collaborators include: Prof Mary Brosnan, National Maternity Hospital, Dr Denise O’Brien, UCD School of Nursing, Midwifery and Health Systems, and Dr Barbra Coughlan, UCD School of Nursing, Midwifery and Health Systems.
Ironmother is a randomised controlled trial comparing alternate day to daily ferrous fumarate (Galfer) in the treatment of confirmed iron deficiency anaemia (IDA) between 12- 34 weeks’ gestation over a 4-week treatment period. This study is being sponsored and overseen by the Clinical Research Centre at University College Dublin.
Iron deficiency anaemia in pregnancy is a worldwide issue linked to poor health outcomes in the mother, fetus and infant. In low- and middle-income countries, the rates of IDA in pregnancy approach 50%. Incidence rates from over 15 European countries vary between 21-35%. Despite treatment being affordable and accessible in the form of ferrous iron salts, compliance with oral iron supplementation can be limited due to unpleasant gastrointestinal side-effects. There appears to be a dose dependent relationship to these side-effects. We hypothesise that alternate day dosing will be as effective as daily iron in correcting iron deficiency anaemia with a more favourable side-effect profile.
This study is clinically and scientifically required to advance the knowledge of healthcare professionals in treating this common problem and to improve the quality of life of women taking iron supplements in pregnancy.
We are developing clinical guidelines and a nutrition checklist that can be used globally to assist healthcare professionals caring for pregnancy women to advise them about appropriate nutrition before, during and after pregnancy
A number of studies have been performed examining the interaction of vitamin D and lipids on maternal and fetal health.
Collaborators:
Dr. Malachi McKenna, Endocrinology, St Vincent’s Hospital, Dublin
Dr Patrick Twomey, Pathology, St Vincent’s Hospital, Dublin
Dr Rachel Crowley, Endocrinology, St Vincent’s Hospital, Dublin
Dr Ciara McDonnell, Paediatric Endocrinology, Tallaght Hospital
Virtual Reality in Medical education
We are developing a virtual reality model of pregnancy to enhance medical and midwifery students experience of learning, these include VR model of fetal development, fetal presentation and insertion of a Bakri Balloon in the management of postpartum haemorrhage. These VR learning environments hold considerable potential to enrich student and trainee learning and to develop practical surgical skills
Collaborators:
Prof Eleni Mangina, UCD School of Computer Science.
Collaborative project with UCD Psychology studying women’s and students’ experiences of bedside teaching. Development and Validation of a questionnaire studying women’s attitudes towards bedside teaching.
Qualitative research of women’s lived experience of a diagnosis of Gestational Diabetes; development and validation of questionnaire studying women’s attitudes towards a diagnosis of gestational diabetes.
Multicentre RCT in aspirin use to prevent pre-eclampsia in women with pre-gestational diabetes.
A baby is stillborn every 16 seconds, leading to heartbreak for more than two million families worldwide per year. Despite advances in care for babies after birth, reduction in the babies dying before birth (stillbirths) are lagging behind. Over 50% of stillbirths are associated with a reduction in baby movements in the womb, but there is currently no way to track baby movements at home.
A team of biomedical engineers led by Prof Niamh Nowlan and clinicians in University College Dublin led by Prof McAuliffe, together with collaborators in Imperial College London and University of Dhaka are developing a unique, wearable baby movement monitoring system for Mum, which they hope will address the urgent need to enable monitoring of baby movements in the womb at home, and dramatically reduce stillbirths globally. This expert team has just been awarded major funding through the Wellcome Leap In Utero Program to determine how this monitor (called FM monitor) can be used as a measure baby’s health in the womb, potentially identifying babies who are at risk of stillbirth, while also offering reassurance when the baby is healthy, thereby decreasing the rates of unnecessary induction of labour and early delivery.
A breastfeeding-friendly city is one where all sectors of the city (healthcare, community and workplace) work together to support breastfeeding. This objective of this project is to develop indicators of a breastfeeding-friendly city. The project is a collaboration between UCD Perinatal Research Centre, RCSI & UCD Malaysia Campus, Technological University Dublin and World Alliance for Breastfeeding Action (WABA).
Dr Canniffe and Prof McAuliffe have led up on the establishment of Cardiac Maternity Registry Ireland. This registry aims to collect data on women with cardiac disease during pregnancy and record on cardiac and pregnancy outcomes. Dr Aoibhinn Smyth is a further member of the team. This is a collaboration with NPEC (national perinatal epidemiology centre) and is set up with a governance structure to monitor data quality and data access. All data is held securely and is anonymised. To date the output of this Registry has results in many presentations to national conferences.
