Freeman Lab Research Topics
Thursday, 15 February, 2024
Immunoengineering
Thursday, 15 February, 2024
The development of chemoresistance presents a major challenge in effectively treating osteosarcoma, and overcoming this challenge is crucial for better patient outcomes. The interaction between cancer and the immune system has long been recognized as a critical aspect of chemoresistance. Tumour cells within the tumour microenvironment suppress antitumor immunity, and immunosuppressive cells and cytokines provide the extrinsic factors for tumour drug resistance. A Stimulator of Interferon Genes (STING) agonist is a promising activator of antitumor immunity.
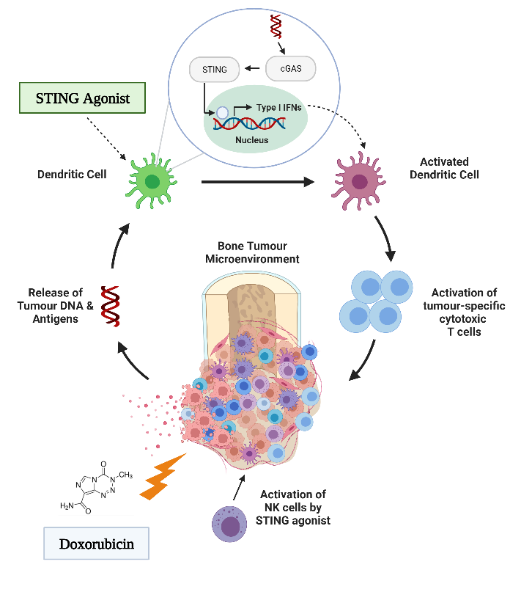
However, while STING agonists are being advanced to pre-clinical trials for several solid tumours, the immunologic and therapeutic impact of STING activation has not yet been explored in the context of osteosarcoma. Our lab is interested in understanding the role of the STING pathway in how the innate immune system recognizes osteosarcoma. This understanding would enable us to develop a therapeutic that targets this pathway, enhancing the therapeutic potential of chemotherapy and preventing chemoresistance.
3D Tumour Models
Thursday, 15 February, 2024
Traditionally, 2D cell cultures have been used as the gold standard in vitro model in cancer research. Although inexpensive and relatively easy to generate and maintain, they do not accurately reflect the solid tumour characteristics and the complex cross-talk between tumour cells and their microenvironment. This has led to persistent failure in translating preclinical drug candidates into successful clinical treatments.
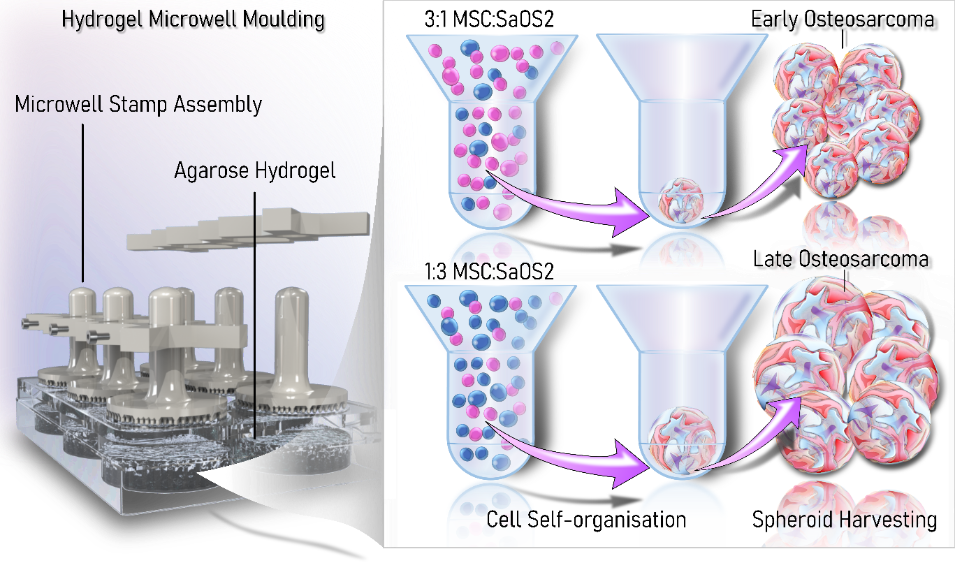
Recently there has been increasing interest in 3D models for cancer as they more closely reflect the phenotypic behaviour of original tumours than conventional 2D cell cultures. This is particularly important for sarcomas, like osteosarcoma, as growth rates, cell morphology, cell–cell junctions, and kinase activation of spheroids have previously been shown to closely mimic those of primary tumours. Our lab has developed a multicellular 3D spheroid model of osteosarcoma which can model early and late-stage osteosarcoma. Our lab is now interested in increasing the complexity of this model through the incorporation of immune cells.
Nanoparticle Delivery
Thursday, 15 February, 2024
Nanotechnology offers a range of tools for both diagnosing and treating cancer. These tools include novel imaging agents and multifunctional devices capable of overcoming biological barriers to deliver therapeutic agents directly to cells and tissues involved in cancer growth and metastasis. Furthermore, these devices have the potential to predict molecular changes in precancerous cells. Our lab is interested in utilizing these novel delivery cargos to transport the treatment directly to cancer cells or the patient's own immune cells, enhancing the patient's ability to attack the tumour itself.

K7M2 (Mouse) Osteosarcoma Cells transfected with flurescently labelled nanoparticles. Actin cytoskeleton stained in green, nanoparticles stained in pink, and nuclueus stained in blue.
3D Bioprinting
Thursday, 15 February, 2024
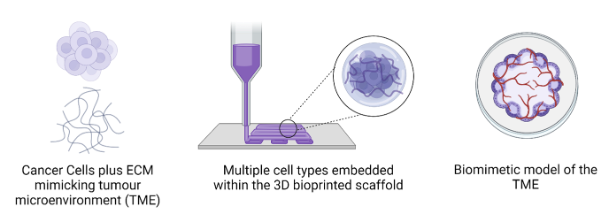
Bioprinting has emerged as a revolutionary technology in the field of cancer research, offering a promising avenue for the development of complex tumour models. This cutting-edge technique allows scientists to create intricate 3D structures that closely mimic the microenvironment of tumours, including their heterogeneous cellular compositions and unique spatial arrangements. By precisely depositing live cells, extracellular matrices, and growth factors layer by layer, bioprinting enables the recreation of tumour models with remarkable accuracy. Our lab aims to use these advanced models not only to gain a better understanding of osteosarcoma tumour biology and behaviour but also to serve as invaluable platforms for drug testing, personalized medicine, and the exploration of novel therapeutic strategies.
Organ-On-Chip
Thursday, 15 February, 2024
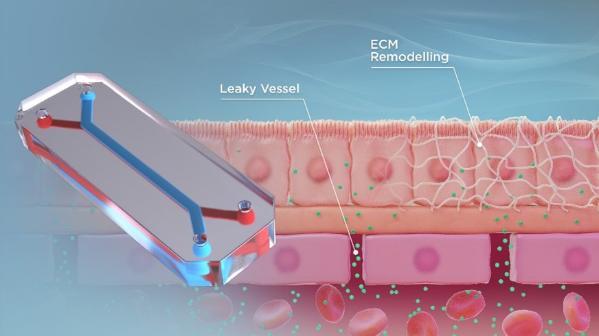
Currently, secondary lung metastasis is the most critical clinical factor for osteosarcoma patients, with 70% of those who develop lung metastasis succumbing to the disease within 3 years. Accelerating cures for patients with poor outcomes remains a challenge, and this is, in part, due to the fact that osteosarcoma is a relatively rare disease. Therefore, recruiting enough eligible participants to conduct clinical trials is a difficult endeavour.
An organ-on-a-chip device is a three-dimensional microfluidic device that recapitulates the complex structures and functions of living human organs. These devices consist of transparent, flexible plastic material, roughly the size of a USB memory stick. Within the device, intricate hollow microfluidic channels are lined with living human cells, 3D tissues, and blood vessels. These living, three-dimensional replicas of human organs offer insights into their inner workings and the effects that drugs can have on them, all without involving humans or animals.
Our lab aims to use these devices to understand why osteosarcoma preferentially metastasizes to the lung and potentially identify a novel therapeutic target or strategy for combating osteosarcoma metastatic disease progression.