Global hydrocortisone trial sees steroid reduce death in sickest COVID-19 patients
Posted 3 September, 2020
An affordable and widely available steroid has been shown to reduce mortality in COVID-19 patients receiving intensive care, new research finds.
Hydrocortisone, an anti-inflammatory drug, was found to improve the recovery of patients following seven days of treatment in 93% of cases compared to those who were not treated with the steroid.
This is according to a worldwide trial, which was overseen in Ireland by (opens in a new window)Professor Alistair Nichol, chair of critical care medicine at University College Dublin.
In a (opens in a new window)paper published in the Journal of the American Medical Association (JAMA), researchers demonstrated that delivering intravenous hydrocortisone, a corticosteroid, improved recovery and survival for critically ill COVID-19 patients.
The findings were made through the “Randomised Embedded Multifactorial Adaptive Platform-Community Acquired Pneumonia” (REMAP-CAP) trial.
The World Health Organisation is now updating its COVID-19 treatment guidance as a result.
"This is really good news for patients who are unfortunate enough to get really unwell with COVID-19 and end up in intensive care units in Ireland,” said Professor Nichol, speaking on (opens in a new window)Newstalk Breakfast.
"Steroids are a really inexpensive drug... [and] the trial that we conducted... showed that if we gave this inexpensive drug to patients when they enter the ICU and are very unwell, it can actually reduce the mortality rate from 40% to 32%.
"So that's a 20% reduction in mortality, and it can also reduce the need for the complex and expensive machines that people sometimes need to support organs when they're unwell".
He added: "I would not recommend to anyone who is listening to this to go out and take steroids if they were unwell, or thought they had symptoms of COVID.
"Steroids are a powerful drug that suppress the immune system, so we wouldn't recommend people to make this decision themselves.
"This study that we did was very much confined to people who are critically unwell in the ICU.”
Between March and June, the REMAP-CAP corticosteroid trial randomized 403 adult COVID-19 patients admitted to an intensive care unit to receive the steroid hydrocortisone or no steroids at all. 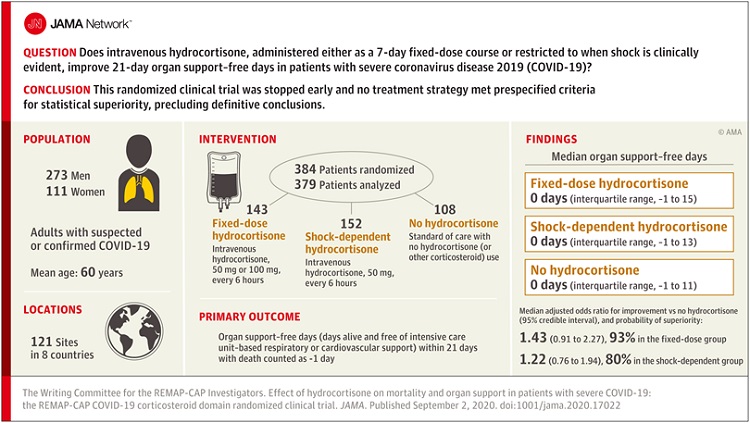
The trial found a 93% probability that giving patients a seven-day intravenous course of hydrocortisone would result in better outcomes, and were consistent across age, race and sex.
The Health Research Board helped fund the Irish arm of the trial which was set up by a group of intensive care specialists from around the world in response to the H1N1 pandemic in 2009.
“Data from REMAP-CAP also contributed by combining data with other corticosteroid trials to confirm these results,” said Professor Nichol.
“This is an important extension of current knowledge that gives clinicians an alternative corticosteroid to use in case of shortages in availability of dexamethasone.”
Dr Mairead O'Driscoll, Chief Executive at the HRB added: “This rapid response to address critical cases of COVID 19 is possible because of a long term investment by the Health Research Board in the Irish Critical Care Clinical Trials Network based at the UCD Clinical Research Centre, and in clinic research facilities and infrastructures across in Ireland.
“Since 2007, the HRB has invested more than €160 million in Ireland's clinical research ecosystem. It is good to see that by having this infrastructure in place, we can help deliver such timely and relevant outcomes for patients.”
By: David Kearns, Digital Journalist / Media Officer, UCD University Relations






