You are invited to participate in a UCD research study, funded by Science Foundation Ireland.
Thank you for taking time to read this.
Study title:
Home monitoring of respiration in COVID-19 patients using smartphone technology.
Investigators:
Dr. Emer Doheny, Insight Centre for Data Analytics and Neuromuscular Systems Research Group, Electrical and Electronic Engineering, University College Dublin
Prof. Madeleine Lowery, Insight Centre for Data Analytics and Neuromuscular Systems Research Group, Electrical and Electronic Engineering, University College Dublin
Dr Silke Ryan, Respiratory Consultant, St. Vincent's Hospital, Dublin and School of Medicine, University College Dublin
What is the purpose of this study?
The aim of the study is to develop a new method to remotely monitor breathing using the microphone in your smartphone. Breathing rate is an important vital sign and is altered in many lung and heart conditions. It is of particular interest in patients suffering from COVID-19. A way to monitor breathing using smartphones will help healthcare providers to monitor patients while they are self-isolating due to Covid-19.
Why am I invited to participate?
You are invited to participate in this study because:
(A) you have been diagnosed with COVID-19
OR
(B) you have not been diagnosed with COVID-19 and you do not have any of the following symptoms of COVID-19
- a fever (high temperature - 38 degrees Celsius or above)
- any kind of cough
- shortness of breath (breathing difficulties)
- loss or change to your sense of smell or taste
- Have you been in close contact with anyone with a confirmed case of COVID-19 in the last 14 days?
What will happen if I volunteer?
Your participation is entirely voluntary. If you initially decide to take part you can subsequently change your mind without difficulty. Furthermore, your doctor may decide to withdraw you from this study if they feel it is in your best interest.
If you agree to participate, you will be requested to record 90 seconds of your breathing using your smartphone microphone. You should position your phone as shown in Figure 1, and try to relax and breathe normally during the recording period.
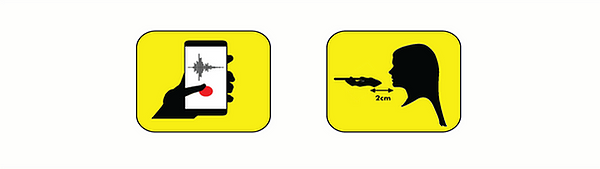
Figure 1. Hold your phone horizontally, with the base of the phone 2 cm from your mouth.
If you agree to participate in a secondary validation test, you will also be asked to perform the 90 s recording, while also wearing a respiratory inductance belt around your chest to measure how you breathe.
After you have made the recording, please email your recording to neuromuscular@ucd.ie. The researchers will save your microphone recording, but they will not save your email address. Your email will be deleted and your email address will not be recorded.
You will also be asked to complete a questionnaire (using google forms). This de-identified questionnaire will:
- Record your age and sex (male/female)
- Record your phone manufacturer and model (e.g. iPhone 5/Huawei P20)
- Record details of any issues/difficulties with your recording
- Record any feedback you have for the researchers
- Check that you do not have any of the COVID-19 symptoms/risks (listed above).
- Confirm that you consent to participate in this study.
Are there any benefits from my participation?
You will not benefit directly from taking part in this study but the information we will obtain may help to develop new remote methods to monitor breathing. These methods may help doctors to better monitor patients within their home using the technology available in smartphones. No analysis of wellbeing or illness will be made from your recording and no feedback will be given.
Are there any risks involved in participating?
There are no risks associated with this study, other than those associated with the day to day use of your phone. The risks associated with mobile phone usage have been summarised for the public by the HSE in their Health Finder on-line service (Risks of Mobile Phone Usage https://www.hse.ie/eng/health/az/m/mobile-phone-safety/). As the breathing data will be recorded as audio files, it is possible that background noises containing personal information could also be recorded. To avoid this, you will have the chance to delete any recordings that may contain this type information and to re-record your data. Your audio recordings will be saved and your email will be deleted.
Your email address will not be saved. Your online questionnaire will be linked to your audio recording, and will not include your name or email address.
What happens if I do not agree to participate?
You are free to decline to participate in this study.
Confidentiality
Your identity will remain confidential. A study number will be used to identify you. Your name will not be published or disclosed to anyone. The researchers will not have access to your personal information and only anonymised data which will be used and stored in this study. All recorded data will be retained by the researchers in a secure location.
Compensation
The researchers are adequately insured by virtue of their participation in the Clinical Indemnity Scheme, or through their research institutions.
Who is organising and funding this research?
This study is organised by the Insight Centre for Data Analytics, University College Dublin in Collaboration with St. Vincent’s University Hospital and patientMpower ltd. The study is funded by Science Foundation Ireland.
All UCD employees involved with this study are subject to Data Protection and Confidentiality measures.
Has this study been reviewed by Ethics Committee?
The St. Vincent’s Healthcare Group, Ethics and Medical Research Committee have reviewed and approved this study.
Contact details
Name: Dr Emer Doheny
Phone: 017162313
Email: emer.doheny@ucd.ie
Research participant's rights
If you have any questions about your rights as a research participant, then you may contact the Hospital’s Quality & Patient Safety Department 01 2214013
Please email any questions to neuromuscular@ucd.ie or emer.doheny@ucd.ie, and we will get back to you as quickly as possible.



