What are you up to, FX06?
Hello there, my name is Zhiran Wang, and I am a PhD student just entering the 2nd-year under the supervision of Prof. Guenther Eissner in SBI. Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been a major health emergency since 2019. There are very limited medication options to prevent the progression from mild to severe illness for COVID-19 patients in clinical settings. My research project is about investigating if a drug, FX06, can help reduce the severity of COVID-19. I produced a decent amount of data during my first year, and I would like to introduce my project and present my research progress in this blog.
Background
Let’s start by understanding why cytokine storms are of interest in the context of SARS-CoV-2 infection. SARS-CoV-2 infection is accompanied by enhanced levels of pro-inflammatory cytokines in COVID-19 patients’ bodies. The excessive release of cytokines is called a cytokine storm [1]. Keeping this in mind, cytokine storm is notorious for causing endothelial dysfunction, for instance, damage to endothelial cell-cell junctions, endothelial inflammation, senescence, etc[2]. The interior surface of the blood vessels and lymph vessels is composed of Endothelial Cells (ECs). Damages to the ECs cause severe complications (i.e., acute respiratory distress syndrome (ARDS), coagulopathy, multi-organ failure, and even death) in COVID-19 patients [3, 4].
The fibrin-derived peptide FX06 is EC-protective in animal models [5]. FX06 was also applied in a small clinical study. Six patients diagnosed with moderate and severe ARDS were mechanically ventilated [6]. After treating them with FX06, pulmonary vascular walls were normalized, and lung functions were preserved. FX06, as the main interest of my project, is a promising drug for protecting COVID-19 patients against progression from mild to severe disease. Until now, the only known target of FX06 is VE-Cadherin/CD144 [6], a type of molecule that helps cells adhere to each other. FX06 prevents leukocytes (white blood cells involved in the immune response) from migrating through by binding to VE-Cadherin/CD144. The protective mechanism of FX06 is still under investigation.
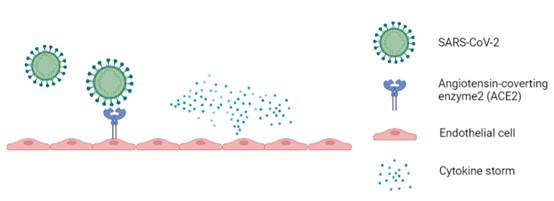
Figure 1. The simplified process of SARS-CoV-2 infection (Created by biorender.com)
Angiotensin-converting enzyme 2 (ACE2) is widely expressed in endothelial cells from multiple organs [7]. SARS-CoV-2 directly infects ECs by ACE2, which causes endothelial activation, cellular damage, and cell death. It has been reported that multiple cytokine levels were amplified in COVID-19 patients [8]. Increased levels of pro-inflammatory cytokines also induce a loss in the normal functioning of ECs [7].
How to mimic the inflammatory status of SARS-CoV-2
We would like to mimic SARS-CoV-2’s inflammatory statuses corresponding to mild and severe illnesses of COVID-19 patients. Ten cytokines that were reported more widely were picked up (IL-1α; IL-1β; IL-6; IL-8; IL-10; IL-18; IFN-γ; TNF-α; IL-1ra; IP-10), and their concentrations were decided based on COVID-19 patients’ serum profiles. We named this selection of cytokines as a Cytokine Cocktail. Once the primary constituents of the cytokine storm were identified, the inflammatory status of COVID-19 was mimicked by treating the ECs with the cytokine cocktail.
Figure 2. The illustration of three major milestones. (Created by biorender.com)
Before I started working on figuring out FX06’s protective effects on endothelial cells, endothelial cells were treated with different concentrations of FX06 for different intervals of time. FX06 didn’t turn out to have significant effects on endothelial cells’ viability
Techniques used in this project
Let me now explain the techniques that I have used and will use during my PhD.
Transendothelial Migration (TEM) Assay enables the investigation of functional changes in the endothelium. More immune cells travel through the endothelium reflecting the increased permeability of the endothelium. I would like to reveal if FX06 can help return the permeability of endothelium damaged by cytokine storm to normal. To check if FX06 is effective, we would need to see a reduction in the number of immune cells that migrate through the endothelium.
I will also be using flow cytometry analysis, a technique used to detect and measure the characteristics of a population of cells or particles. The particles/cells are suspended in a fluid and placed in the flow cytometer for analysis. I will use it to measure the percentage of dead ECs after cytokine exposure with or without FX06, and the immunological changes (i.e. expression levels of surface markers) that have occurred.
This project also employs bioinformatics to figure out changes in the transcriptome and proteome of ECs, to give us more information on RNA and protein expression levels.
Revealing functions of FX06
To figure out if FX06 assists in maintaining the permeability of endothelial barriers in the situation of cytokine storm triggered by COVID-19, I utilized the TEM Assay.
First, I cultured endothelial cells to let them form a monolayer. A monolayer is a single layer of cells such that one cell doesn’t grow on top of another. Later, the endothelial monolayers were treated according to my experimental design. In the end, PBMCs, a population of immune cells derived from healthy human donors were added on top of the endothelial cells. After a certain time, the number of PBMCs that migrated through the endothelial monolayer was counted. To achieve cell counting in this assay, I used a High Content Screening Confocal Microscope to do this tricky job.
The microscope took multiple image stacks in a large depth range from all samples rapidly. I would find the monolayer by myself among all those images, so any cells below the endothelial monolayer represents those that migrated through the monolayer. A software application was used to count the total number of PBMCs. My results have shown that the cytokine storm increased the number of PBMCs that migrated through the endothelial barriers, whereas FX06 showed protective effects by decreasing PBMC migration caused by the cytokine storm.
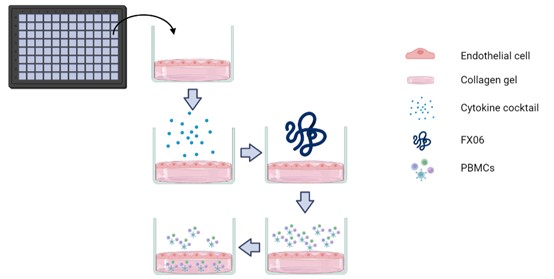
Figure 3. Illustration of TEM assay. (Created by biorender.com)
The TEM assay with three conditions, the control group, cytokine cocktail treated group, and cytokine cocktail plus FX06 treated group. Endothelial cells were cultured on top of collagen gel. After endothelial cells formed monolayers, cells were treated with the cytokine cocktail for 24 hours. The cytokine cocktail plus FX06 treated group was treated with FX06 for 2 hours after the treatment of the cytokine cocktail.
PBMCs isolated from healthy donors were added to endothelial cells. After 18 hours, the High Content Screening Confocal Microscope took images for all groups to count the number of PBMCs that migrated into the collagen gel.
To reveal how FX06 exerts protective effects on endothelial cells in the context of cytokine storms, mass spectrometry analysis was conducted. To do this, HULEC-5a cells, microvascular endothelial cells from the lungs, were treated with the cytokine cocktail for 24 hours and treated with FX06 afterwards for 6 hours. Proteins whose levels increased after treatment with FX06 in HULEC-5a were associated with negative regulation of blood coagulation, compared to cytokine cocktail-only-treated HULEC-5a. Angiopoietin-related protein 4 (Angptl4) was found to have increased levels after FX06 treatment. Angptl4 was reported to reduce vascular leakage and the overexpression of Angptl4 helped normalize the endothelium's permeability[9, 10]. Compared to FX06-treated HULEC-5a, proteins that were upregulated in cytokine cocktail-treated HULEC-5a were involved in leukocyte transendothelial migration, cytoskeleton changes, etc.
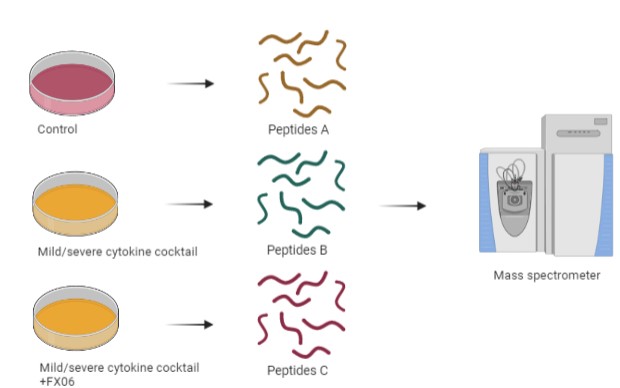
Figure 4. Workflow of Mass Spectrometry Analysis. (Created by biorender.com)
It aimed to disguise how FX06 exerted changes on endothelial cells in the context of a cytokine storm of COVID-19 in a protein expression level.
Future plans
In the next two years of my PhD, I will continue investigating cell death and immunological changes (expression levels of surface markers) of endothelial cells in the situation of SARS-CoV-2 inflammatory status and FX06 treatment via flow cytometry analysis. Some markers are related to endothelial integrity, while others are associated with adhesion and migration processes. Flow cytometry analysis would give me a more detailed and accurate view of how cells behave. In addition, we would like to continue with mass spectrometry analysis by adding more time points (i.e., FX06 treatment of 5 minutes, 20 minutes, 2 hours, and 6 hours). I wish to thoroughly examine the behaviour of FX06 against COVID-19-triggered cytokine storm.
The project is funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Health and Digital Executive Agency (HADEA). Neither the European Union nor the granting authority can be held responsible for them.
References
- Ragab, D., et al., The COVID-19 cytokine storm; what we know so far. Frontiers in immunology, 2020: p. 1446.
- Xu, S.-w., I. Ilyas, and J.-p. Weng, Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacologica Sinica, 2022: p. 1-15.
- Bonaventura, A., et al., Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nature Reviews Immunology, 2021. 21(5): p. 319-329.
- Norooznezhad, A.H. and K. Mansouri, Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (COVID-19). Microvascular research, 2021. 137: p. 104188.
- Adam, E.H., et al., Fibrin-derived peptide Bβ15-42 (FX06) as salvage treatment in critically ill patients with COVID-19-associated acute respiratory distress syndrome. Critical Care, 2020. 24(1): p. 1-4.
- Wu, M.A., et al., COVID-19: the key role of pulmonary capillary leakage. An observational cohort study. 2020, medRxiv.
- Zhang, J., K.M. Tecson, and P.A. McCullough, Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Reviews in cardiovascular medicine, 2020. 21(3): p. 315-319.
- Xiao, N., et al., Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nature communications, 2021. 12(1): p. 1-13.
- Wang, Y., et al., Effect of angiopoietin-like protein 4 on rat pulmonary microvascular endothelial cells exposed to LPS. International Journal of Molecular Medicine, 2013. 32(3): p. 568-576.
- Ito, Y., et al., Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer research, 2003. 63(20): p. 6651-6657.

