Professor Paula Bourke
Research
- Awards and Prizes
- Research Themes
- Research Institutes
- Research Centres
- Spin Out Activity
- ERC Grants
- Technology Transfer
- International Networks
- Horizon Europe
- Postgraduate Student Opportunities
- Industry Collaborations
- Entrepreneurship Training
- 2024 STEM Symposium
- The UCD STEM Challenge Fund
- Smurfit Kappa Newman Fellowship Award
- Impact Showcase
- Will Dimond & Marcus Donaghy
- Dr Oliver Kinnane
- Dr Elizabeth Shotton
- Professor Madeleine Lowery
- Dr Simon Kelly
- Dr Eoin O’Cearbhaill
- Professor Francis Butler and Dr Kevin Hunt
- Professor Paula Bourke
- Professor Aoife Gowen
- Professor Da-Wen Sun
- Dr Fionnuala Murphy
- Dr Tom Curran
- Dr Michael Long
- Dr Yaqian Zhao
- Dr Sarah Cotterill
- Dr Fiachra O’Loughlin
- Professor Eugene O’Brien
- Dr Shane Donohue
- Professor Eoin Casey
- Dr Philip Donnelan
- Professor Niall English
- Dr Mohammad Reza Ghaani
- Dr Elena Blokhina
- Dr Damian Flynn
- Professor Andrew Keane
- Professor R. Bogdan Staszewski
- Dr Le-Nam Tran
- Professor Eoin O'Neill
- Dr. Liana Ricci
- Dr Aisling Ní Annaidh
- Professor Lizbeth Goodman
- Dr Kevin Nolan
- Dr. Nan Zhang
- Dr Francesco Pilla
- Professor Mark Scott & Dr Michael Lennon
- Wall of Frames 2024
New Approaches to Plasma Therapy may Improve Medical Implant Outcomes

Professor Paula Bourke - UCD School of Biosystems & Food Engineering
The race is on to find new and effective ways of treating antimicrobial resistant pathogens, which represent a major threat to the health of patients undergoing surgery for medical implants. Bacterial infection is one of the most common problems associated with such surgery, compounded by the absence of new antibiotics to take up the battle. But a tripartite team involving researchers at UCD and QUB and at Jefferson University in Philadelphia are pioneering new approaches. Plasma is the fourth state of matter (the others are solid, liquid and gas. The researchers are using a combination of the direct application of cold plasma and plasma functionalised liquids. Liquids such as water or saline, can be exposed to a plasma in order to generate particular chemical characteristics, and are under investigation for a range of applications including infection control and cancer therapy. Although still in its early days, if the research is successful it could revolutionise outcomes, vastly improving the quality of life for hundreds of thousands of people each year and providing a new tool in the ongoing battle against antibiotic resistant microorganisms.
The use of plasma is quite promising because it does not rely on one particular mechanism of action. So understanding how to best use the different disruptive mechanisms is what we hope to achieve.
A Problem Crying Out for a New Solution
Orthopaedic implants have been one of the major advances of modern medicine, eliminating or reducing pain and enhancing mobility and quality of life for millions of people each year. But insertion of these devices into the human body can be associated with complications, and one of the most common is bacterial infection. This is a large and growing problem because of the emergence of drug-resistant pathogens at a time when the clinical pipeline of new antibiotics is running dry. Of the 32 antibiotics in clinical development in the WHO’s list of priority pathogens in development in 2019, only six were classified as innovative.
However, one research project currently underway in the School of Biosystems & Food Engineering seeks to harness the power of plasma as an alternative and/or complementary approach to the use of antibiotics in preventing and treating device-associated infections. The tripartite project has been funded under a tri-partite awarding arrangement, following the Good Friday agreement, with UCD in the Republic, Queen’s University Belfast in Northern Ireland and Jefferson University in Philadelphia as its three key partners.
Plasma is the fourth state of matter (the others are solid, liquid and gas) and is the result of supplying energy to a gas. These partially or completely ionised gases make up over 99% of the observable universe and can be seen in such phenomena as lightning and the aurora borealis.
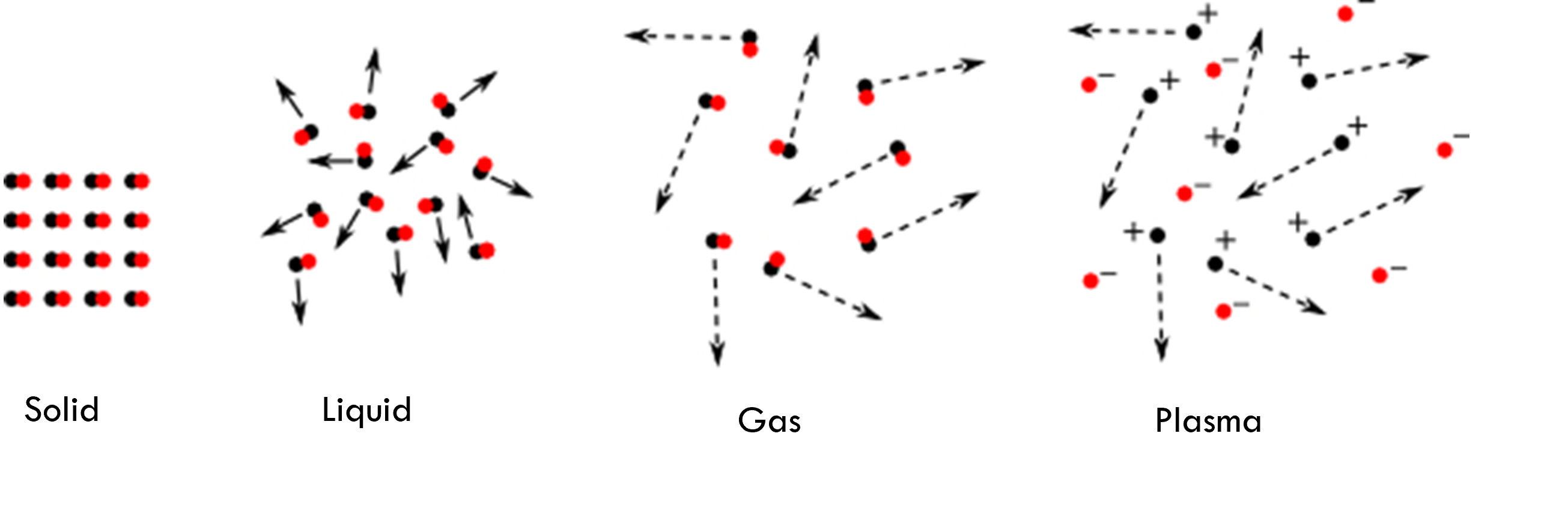
But in recent decades “cold plasma” which can be generated at atmospheric pressure and with temperatures that are tissue tolerable, has opened up exciting new possibilities in the fields of food safety and biomedical applications.
A Conway Institute Research Fellow and a member of the UCD Institute of Food and Health, Professor Paula Bourke is one of five UCD professors ranked among the top 1% of the most cited researchers in the world according to the 2020 Highly Cited Researchers report, compiled by Clarivate Analytics. Her initial interest some six years ago was in the potential role of plasma functionalised liquids in treating foods and different biomaterial samples. “Most of the new technologies I develop are used in food processing or agricultural situations,” she explains. “One of the key questions we ask is whether a novel approach can be safely used and what might the unknown, longer-term effects be? In a previous project within the EU’s Seventh Framework, the Commission asked us to investigate whether it was safe to use plasma technology to decontaminate food in order to enhance its shelf life. We developed a way of looking at the short term and the longer-term safety profile of applying these technologies.

Cold plasma processes are also being developed for use with foods such as grains and seed
Arising from this, Prof Bourke and her team began looking for a way of retaining the anti-microbial effect without producing something too toxic to other types of cells. The findings of this work, presented at the 6th International Conference for Plasma Medicine in 2017, attracted attention from others working with plasma. One of these was Dr Terry Freeman, a professor of orthopaedic surgery at Jefferson University in Philadelphia and a pioneer in the field already using plasma therapy to try to promote wound healing and bone regeneration.
Dr Bourke had already been collaborating with a colleague in Northern Ireland, Professor Brendan Gilmore of QUB’s School of Pharmacy, and they had just prepared a UK/Irish joint funding proposal for research into the microbiological interactions of cold plasma, using a combination of dry and liquid approaches. The Environsafe project, “Plasma Innovations for Food Safety and Sustainability” primarily focuses on major risks to food safety and quality in the food chain, including biofilms (structures formed by bacteria in food and food processing environments as well as on surfaces of implanted medical devices), allergens (a substance that causes an allergic reaction) and toxin producing microorganisms.
Putting the One Health Agenda into Action
A strong advocate of the One Health agenda, which recognises that people’s health is closely connected to those of animals and the environment, when Bourke was approached by Prof Freeman she was open to the idea of combining their various approaches to achieve common goals. Based on the work undertaken over the preceding years into the application of plasmas to create an antibacterial liquid, the tripartite consortium is now exploring cold plasma as a therapy for bone infection prevention and control. The researchers hope to develop a novel approach that will support and provide an added benefit to current therapies.
The researchers are using plasma-generating medical devices that are already approved for use in clinical therapies. They are also looking at combining different approaches to develop a useful therapy. “A therapy can comprise a sequence of steps that can control the problem,” Prof Bourke points out.
Another important aspect of this work involves exposing a liquid such as water or saline to a plasma in order to generate particular chemical characteristics in the liquid. The aim is to create and capture the ability within the liquid to kill antibiotic-resistant pathogens but also to kick off other stimulatory and beneficial effects.
“We can track the chemical changes within a plasma treated liquid and we can end up with a very useful profile. Saline is already widely used in clinical settings for rinsing so it is interesting to see if liquids such as saline can have their functionality boosted. We are attempting to increase the ability of a liquid to have an antibacterial effect at a surgical wound site. Combining this with a gaseous plasma treatment might help us improve control of device associated infection, particularly in extremely vulnerable patients.
“The use of plasma is quite promising,’ she says, “because it does not rely on one particular mechanism of action. So understanding how to best use the different disruptive mechanisms is what we hope to achieve. And it is not necessarily something that must stand alone, either. It could conceivably improve or extend the usefulness of existing antibiotic therapies.”
Significant Potential Impacts
The global implantable medical devices market was worth US$96.6 billion in 2018, with an increase in the number of age-related diseases, concurrent with a rise in life-span, further increasing demand for various life prolonging medical aids including implantable devices. That demand is expected to continue to grow strongly, driven by an ageing population. In Europe, it has been estimated that antimicrobial resistance costs health services more than €9 billion every year. In the US it is estimated to add $20 billion in direct healthcare cost and a further $35 billion in loss of productivity each year. But against the background of a continued increase in drug-resistant pathogens and a decline in the pipeline of new antimicrobials to offset them, any effective new approaches to the treatment of microbes of all kinds arising from such surgeries will contribute hugely to improved health outcomes across society.
Research References
- Paula Bourke, P.; Dana Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K.M. The Potential of Cold Plasma for Safe and Sustainable Food Production Trends in Biotechnology 36(6) DOI:10.1016/j.tibtech.2017.11.001
- Lu, P, Ziuzina, D, Cullen, PJ, Bourke, P*. Inner surface biofilm inactivation by atmospheric pressure helium porous plasma jet. Plasma Process and Polym. (2018). 15:e1800055, https://doi. org/10.1002/ppap.201800055
Media and Social Media
- (opens in a new window)https://nexus.jefferson.edu/science-and-technology/a-passion-for-curiosity
- (opens in a new window)https://www.innovationnewsnetwork.com/the-social-and-economic-consequences-of-antimicrobial-resistance/5812/
- (opens in a new window)https://www.sciencediplomacy.org/perspective/2014/trilateral-partnership-for-supporting-research-and-relationships
- https://www.ucd.ie/research/news/2020/ucdresearcherco-leadonprojecttodevelopnewtherapiestotreatorthopaedicinfection/body,543712,en.html
- (opens in a new window)https://www.sfi.ie/research-news/news/us-ireland-investment/
- (opens in a new window)http://www.opnews.com/2020/07/university-college-dublin-researcher-to-co-lead-a-e4-5m-collaborative-project-to-develop-new-therapies-to-treat-orthopaedic-infection/16399
- https://www.ucd.ie/newsandopinion/news/2020/november/18/ucdresearchersnamedamongworldsmostinfluential/
Acknowledgements
- SFI US-Ireland Research Partnership Programme award registered 2020 for the project entitled ‘Plasma-based therapies for bone infection’
- SFI-BBSRC Research Programme award registered 2020 for the project entitled ‘EnvironSafe: Cold Plasma Innovations for Food Safety and Sustainability’